

|
Collaborate with MS-MRI
Since the summer of 2010, we have processed more than 2,000 MS cases. We are constantly data mining these images and looking for important physiological data on MS and CCSVI. We invite you to send any relevant information such as research papers on MS research that you think may be of help related to the CCSVI theory. Please also read the history section on this web page.
As paraphrased by Dr. George B. Hassin of Chicago in 1935, when reviewing the more risky treatment of MS with sympathectomies: "...multiple sclerosis is such a distressing, hopeless condition that any therapeutic measure that appears promising should be given careful consideration, regardless of the difference of opinion as to the pathophysiologic features of this disease" [Wetherall]. Wetherell F. Cervicodorsal sympathectomy in multiple sclerosis. Arch Neurol Psych 1935; 34: 99-110. |
|
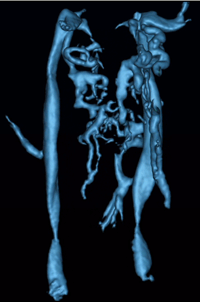
An overview of current perspectives related to the role of the venous vasculature in multiple sclerosis According to Putnam (1) who discussed vascular abnormalities in MS in 1936, the first observations related to abnormal vasculature or effects related to the vasculature appeared in Cruveilhier (2) in 1839, more than 170 years ago who compared areas of sclerosis with the results of embolism. Rindfleisch (3) noted in 1863 an engorged vessel in the center of a plaque and in the same year Charcot (4) described vascular obstruction in MS. These observations would be noted again and again over the next 135 years. What was missing was the advent of imaging as a tool to investigate the vascular system in three dimensions, something that ultrasound takes a step toward as used by Zamboni and more recently the use of magnetic resonance imaging in the study of cerebro-spinal vascular insufficiency or CCSVI. But Putnam didn't stop there. With an ingenious idea, he proceeded to study the effects of obstructed venous flow in the cerebral veins of dogs. These animals developed a number of abnormalities many of them similar to encephalitis or multiple sclerosis. His comment at the end of his paper was as follows (5): "The later stages (up to ten months) of the lesions consist of plaques of demyelinization with practically complete preservation of the axis-cylinders and with dense fibrous gliosis confined to the white matter." And he continues with: "The similarity between such lesions and many of those seen in cases of multiple sclerosis in man is so striking that the conclusion appears almost inevitable that venular obstruction is the essential immediate antecedent to the formation of typical sclerotic plaques." Despite the wonderful immunological advances in the last 75 years, how can we ignore the early work that so clearly demonstrates the role of the venous system in MS? The precocious work of these early researchers today finds its laurels in the current extracranial obstructions proposed by and seen by Zamboni (6) and now others. There are more intriguing connections as one reads these old papers. Venous blood is more prone to clot. And these micro-thrombi may rapidly disappear and so become unobservable. Putnam also observed clotting and perivascular hemorrhage in encephalomyelitis (1). He continued to believe that clotting was a problem for the rest of his professional life (7). An increase in capillary density also seems to develop and this may well describe the "capillary recruitment for venous drainage hypothesis proposed by Haacke". In this hypothesis, the obstructed flow leads to endothelial damage (8) and iron build up (9) and the need to increase the outflow capacity in the venous system. The brain then recruits capillaries to become veins and these in turn are also damaged leading to further iron deposition. If this is the case, then the iron build up should take place backward along the venous drainage system, which appears to be the case (10). The story continues with a reference to Borst who founded a theory on the occurrence of vascular obstruction where he mentions the process of significant narrowing to the point of complete obliteration, hyaline transformation, etc. Perivascular hemorrhages were also frequently described by many authors. Borst (11) also mentions the presence of pigments. Others describe the combination of all three: congestion, perivascular hemorrhage and pigments (possibly hemosiderin or iron related (1,12)) in encephalitis post measles (13). Many noticed venous engorgement and one study showed that thrombi were visualized in nine of seventeen MS cases and all three encephalomyelitis cases. Could this hemosiderin correspond for example to those cases where we see iron deposition with SWI in lesions in the brain (14)? Three interesting papers (15-17) point toward other features associated with venous congestion, small thrombi and iron deposition. In the first two, children with early cerebral infarction (15) and children following severe ischemic-anoxic events (16) showed increases in iron content in the basal ganglia, thalami and white matter. Iron deposition was also associated with periventricular gliosis (16). In fact, desferrioximine, an iron chelator, has been used to minimize the damage for patients undergoing cardiac resuscitation (18). These findings also may be consistent with the fact that some MS lesions show iron build up. It has not been shown yet but one might conjecture if the MS lesions with the highest iron content may represent ischemic tissue of lesions and may correspond with the lowest blood volume. An interesting case of venous congestion shown with SWI that was similar in appearance to a developmental venous anomaly, also in a child, shows that SWI may be able to detect small thrombosed veins. In this particular case, the child was treated and recovered and evidence of the problem on imaging disappeared at two months (17). So iron regulation appears to be disrupted in ischemic-anoxic insult. Could this be what is happening in MS as well? A very recent paper by Zamboni talks about the increases in iron seen in chronic venous disease for patients who are carriers of the HFE gene's mutation C282Y and H63D (19). Evidently in these people, the intracellular iron deposits of mutated macrophages have less stability than those of the wild type. The diapedesis of red blood cells and the ensuing extracellular hemolysis leads to the release of free iron, which may act as an inflammatory agent. The predominant cells migrating into the extracellular matrix are then T-cells and macrophages. If these macrophages are not functioning properly or the form of ferritin created is corrupted and the iron is prematurely released, then iron may well play a strong role in generating further free radicals. According to F. A. Schelling's tome on the role of the vasculature in MS (20), and lesion genesis, there are a number of crucial observations from which we quote two here. The first relates to the mechanical nature of the problem and the fact that the vascular damage follows a path opposed to the flow. In turn, he quotes Carswell as saying: "In inflammation, the local congestion commences in the capillaries, afterwards extends to the small veins, but never to large branches; in mechanical congestion (by venous flow inversion) the blood accumulates first in the venous trunks, which are always conspicuous, and afterwards in the branches and capillaries (21)." Further evidence of this mechanical effect comes from observations of I.V. Allen who noticed the wide vascular beds around veins and the central widening of the venous tree indicative of intermittent increases in cerebral pressure (22). But it is worth returning to Fog's work where he summarizes his results from a series of cadaver brain studies (23) as "30 plaques showed that they definitely followed the course of the veins, so that course and dimensions of the veins determine the shape, course and dimension of the plaques." He also closes with the comment: "Consequently, multiple sclerosis, pathologico-anatomically, must be considered a periphlebitis, as proved by the author in 1948 in the case of plaques of the spinal cord (24)." As for vascular insufficiency, Putnam discusses this in his 1953 paper (25) entitled: "Cerebral vascular insufficiency" although this paper is about arterial insufficiency. However, he shows that the effects of hypotension can cause significant neurological problems when it leads to a deficit of blood flow to the brain in the presence of already compromised (narrowed) vessels. Another key issue is the role of vitamin D in multiple sclerosis. Perhaps vitamin D plays a role in the health of the endothelium or cardiovascular health in general. A recent paper by Cecik and Stein (26) states: "Vitamin D-deficiency has been associated with many systemic disorders, including infectious, inflammatory, and autoimmune conditions, cardiovascular disease, hypertension and atherosclerosis, neuromuscular function, cancer, neurodegenerative diseases, and neuropsychological and functional outcomes in the elderly population." Further, Nemerovski et al (27) in a review of much literature on the topic state that "vitamin D deficiency was implicated in several types of vascular disease including peripheral artery disease, atherosclerosis, myocardial infarction and ischemic stroke." They also state that vitamin D deficiency has been associated with increased hypertension. Recently it has been demonstrated that there is reduced perfusion and even loss of small medullary vein visibility in multiple sclerosis (28). The idea of reduced perfusion emanates from the work of Putnam as described earlier. A paper by Juurlink has a nice discussion of the role of hypoperfusion in MS (29). He comments that the reduced perfusion can be detrimental to oligodendrocytes, can preferentially affect white matter, causes demyelination and leads to microglial activity. He notes these can be most marked in the optic nerve and tract. He then quotes: "There is now ample evidence that ischemic insults of sufficient severity can cause upregulation of cell adhesion molecules onto the endothelial cells, thus allowing infiltration of leukocytes into the brain parenchyma resulting in an inflammatory lesion." He goes on to point out that hypertension of genetically susceptibility lesions (remember the effects of reduced vitamin D can lead to increased hypertension) that leads to vascular damage then leads to ischemia. It has been long thought that iron misregulation is associated with neurodegenerative disease. There is an extensive recent review of iron in neurodegenerative diseases by Kell (30). Although this will require much more detailed experimentation (30) there is certainly some evidence for it in specific disease such as neuroferritinopathy, aceruloplasminemia and hemochromatosis, for example. In the latter case, Thomas and Jankovic (31) stated: "The presence of central nervous system superficial siderosis and central nervous system vasculitis, in association with systemic hemosiderosis, may be the neurological manifestation of hemochromatosis." (In hemochromatosis, iron levels are sometimes reduced with either chelation or with phlebotomy.) They go on to note that iron elevation follows dopaminergic cell death. On a different but related note, there is some suggestion that the amount of stored iron might also play a role in risks of white matter damage post injury (31). Sullivan suggests that there is evidence that oxidative DNA damage as measured by 80HdG correlates with the amount of stored iron. A very interesting paper by Patt et al (33) and another by Grant et al (34) both suggest that reduced iron levels are associated with reduced damage to the brain. The former reports that: "Gerbils fed a low iron diet for 8 weeks had decreased brain and serum iron levels, less neuroloigical deficits and decreased brain edema after temporary unilateral carotid ligation (ischemia) and then reperfusion than gerbls fed a control standard of iron diet." The latter reports that experimental automimmune encephalomyelitis (EAE) did not develop in low iron mice. They also suggest that: "The mechanism of EAE inhibition in iron deficient mice likely involves the delivery and metabolism of iron for optimal CD4+ T-cell development." In their paper they also comment that iron supplementation has been shown to increase progression and mortality in HIV-infected people and that iron chelation in mice with EAE also reduced the clinical severity of the symptoms. Clearly, iron plays some role in neurological processes that lead to neurogdegenerative effects. But white matter is not the only tissue affected in MS. Derfuss et al (35) have observed infalmation of gray matter blood vessels after transferring TAG-1-spefcific T cells into rats a finding absent in classic models of EAE. When combined with a two hit model using antibodies against myelin, they observed widespread demyelination in both white matter and gray matter. Rudick and Trapp (36) point out that there are three patterns of lesions, I: lesions involving both gray matter and white matter; II: lesions involving perivascular areas of cortical demyelination and III: lesions involving bands of cortical demyelination below the pial surface. Modern pharmaceutical drugs and their effects on the vasculature Perhaps the recent results on CCSVI can actually serve to draw our attention to those aspects of current drug treatment that may be affecting the vasculature in a positive way when the drugs work. Our focus in this section is on the role of cerebral endothelial cells (CEC) in the pathogenesis of MS (37). The blood brain barrier (BBB) creates an impermeable barrier to most of the circulating substances in the blood. It is already assumed by some researchers in MS that the disease is characterized not only by demyelination but also the presence of peri-venular activated leukocytes. Although the following discussion is understood to be a potential pathway to the chemistry of the neurodegenerative process, there is no initiation or known antigen that starts the process. The current belief is that CD4+ T cells become activated toward myelin specific proteins and trigger a massive inflammatory cascade that eventually leads to transendothelial migration of activated leukocytes and macrophages from the vascular space into the brain. CECs themselves may act as antigen presenting cells (APC) presenting antigens to activated leukocytes and acting as human lymphocyte activation (HLA) class II molecules. It is then believed that CD4+ T cells, B cells and macrophages enter the CNS through the disrupted BBB. The initial capturing of the leukocytes into the endothelium involves a rolling of the leukocytes along the underlying endothelial layer. Capture and rolling appear to be necessary for firm adhesion of the leukocyte to the underlying endothelium. This process involves the expression of integrins which bind to its ligand on the endothelial cell (VCAM-1 = vascular adhesion molecule -1). Platelet endothelial cell adhesion molecule-1 (1/PECAM-1) is involved in the regulation of extravasation of activated leukocytes. Elevated levels are seen in MS patients. Also, serum pro-inflammatory cytokines are elevated before clinical exacerbations of MS and these affect CEC and alter CNS endothelium barrier function (38). Elevated serum levels have been seen in MS patients. Matrix metallopoteinases (MMPs) also lead to disintegration of the BBB. MMP-9 levels are elevated in MS (39) and appear to correlate with the degree of BBB disruption (documented by the presence of contrast-enhancing lesions). Interferon may help reduce these BBB disruptions. Specifically, IFN-b (Betaseron, Rebif, and Avonex) adhesion to cell surface receptors may help stabilize CECs by blocking the release of endothelial microparticles (EMP) and transendothelial mirgration of moncocyte-EMP complexes and maintaining expression of junctional proteins (40). Developmental venous anomalies and intracranial venous hypertension Venous hypertension may be a cause of other diseases as well and in some ways similar to what is seen in MS. For example, it is thought to play a role in developmental venous anomalies and may be a cause of intracranial vascular malformations (41). Increased evidence suggests that the primary pathogenetic factor in the development of dural arteriovenous malformations (DAVMs) is venous hypertension related to either thrombotic or nonthrombotic reduction of venous outflow (42-45). Several events that increase venous pressure such as developmental anomalies of the venous system, venous thrombosis, and head trauma or transcranial surgery serve as a trigger (46). Further, cessation of venous hypertension by complete recanalization of the thrombosed sinus may lead to spontaneous cure of the disease. On the other hand, progressive thrombosis or occlusion of venous hypertension leads to an aggressive clinical course (47). Some authors suggest that AVMs in deep locations, such as basal ganglia or in the periventricular or intraventricular space have an increased risk of bleeding. (48,49). This may occur due to the fact that the veins of central drainage have one final common pathway which is the vein of Galen and the straight sinus, opposite to the superficial veins that have more connections and are probably more flexible in adaptation. Venous reflux into a sinus or a deep vein seems to be positively correlated with an increase risk of hemorrhage (50). The clinical presentation of DAVMs has been classified as either benign or aggressive. They are considered benign if they produce only ocular symptoms, pulsatile tinnitus, bruit, and/or local cranial deficits. Most of the symptoms are related to venous overload of the primary draining veins, mainly the superior ophthalmic vein and the inferior petrosal sinus. A more aggressive natural history and more severe clinical presentation are associated with retrograde venous drainage and venous drainage into leptomeningeal veins. (Djindjan and Merland 1978). Some studies show that some DAVMs may disappear without treatment (51-55). Spontaneous closure of cavernous sinus DAVM is frequently preceded by transient worsening of the symptoms, including retinal hemorrhages and reduced vision. Central retinal thrombosis has been implicated as the underlying cause (56,57). A significant change of the symptoms including improvement such as cessation of tinnitus may indicate spontaneous closure. Classification systems for the retrograde venous drainage have been proposed by Djindjan et al (42) and Cognard et al (58). In their analysis, Cognard et al (58) found hemorrhage as the most severe complication was related strictly to cortical venous drainage and was found in 40% type 3 in his classification (veins draining exclusively into cortical veins) and 66% in type 4 (draining into cortical vein with venous ectasia). The significant difference between these 2 types demonstrates that venous ectasia is associated with particularly high risk of hemorrhage, due to vessel wall degeneration that has been found in these venous pouches (59). Borden created a simplified classification system combining the previous two (60). Leptomeningeal venous drainage, venous dilatations and galenic drainage were found to significantly correlate with aggressive symptoms (61). Analysis of the venous drainage pattern demonstrated that high incidence of aggressive symptoms in certain locations was related to the typical venous anatomy in each location rather that to the location itself. Studies done by Davies et al. that support the concept that the type of venous drainage not only determines the first clinical presentation but also predicts reliably the natural history of the AVMs. Of significance to MS patients undergoing treatment are the studies of idiopathic intracranial hypertension. In this group of patients, it has been shown using none other than MR venography that on the order of 90% of these patients have sinovenous stenoses (62). The treatment for stenoses in the transverse and sigmoid sinuses varies but it is possible to open these vessels up again. It is not understood if the hypertension causes these stenoses (63,64), in fact that is one theory, or if it is the other way around. In any case, treating the stensoses tends to relieve the symptoms (65). There are a variety of different methods used to image these abnormalities including CTA, but MRV appears to be the safest and provides sufficient information to make the diagnosis (67). References (1) Putnam (1937). Evidences of vascular occlusion in multiple sclerosis and encephalomyelitis. Arch. Neurol. Psychiatry 6: 1298-1321. (2) Cruveilhier (1835-1842) Anatomie pathologique du corps humain. Paris, Bailliere, Vol 2. (3) Rindfleisch (1863). Histologisches detail zu der grauen degeneration von gehirn und rueckenmark. Arch. Path. Anat. Physiol. Klin. Med. 26: 474. (4) Charcot (1868) Histologie de la sclerose en plaques. Gaz Hopit Civils Milit, 41: 554-566. (5) Putnam (1935). Studies in multiple sclerosis: encephalitis and sclerotic plaques produced by venular obstruction. Archives of Neurology and Psychiatry. 33: 929-940. (6) Zamboni (2009). Chronic cerebrospinal venous insufficiency in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 80:392-399. (7) Rowland (2009). The legacy of Tracy J. Putnam and H. Houston Merritt. Oxford Univ. Press, page 142. (8) Adams (1987). Periventricular lesions in MS. Neuropathol Appl Neurobiol. 13: 141. (9) Singh and Zamboni (2009). Anomalous venous blood flow and iron deposition in multiple sclerosis Anomalous venous blood flow and iron deposition. JCBF 1-12. (10) Haacke et al. (2010). Evidence of an increase in basal ganglia and thalamic iron content in multiple sclerosis and its vascular implications: An initial analysis with susceptibility weighted imaging. Submitted to Intern. Angiology. (11) Borst (1903). Die multiple sklerose des zentralnervensystems. Ergebnisse Allg Path Pathol Anat, 9: 67-187. (12) Spatz (1921-1922). Zur eisenfrage, besonders bei der progressiven paralyse. Zentralbl. f. d. ges. Neurolo. u. Psychiat. 27:171. (13) Wohlwill (1928). Ueber encephalomyelitis. Neurol and Psychiat, 112: 20. (14) Haacke et al (2009) Characterizing iron deposition in multiple sclerosis lesions using susceptibility weighted imaging. J Magn Reson Imaging. 29:537-44. (15) Cross et al (1990). MR evaluation of brain iron in children with cerebral infarction. AJNR 11; 341-348. (16) Dietrich et al (1988). Iron accumulation in the basal ganglia following severe ischemic-anoxic insults in children. Radiology 168; 203-206. (17) Amemiya et al (2008). Venous congestion associated with developmental venous anomaly: Findings on susceptibility weighted imaging. JMRI 28: 1506-1509. (18) Gutteridge et al (1979). Inhibition of the iron-catalysed formation of hydroxyl radicals from superoxide and of lipid peroxidation by desferrioxamine. Biochem J 184; 469-472. (19) Zamboni et al (2008). Inflammation in venous disease. Int Angiol 5; 361-369. (20) Schelling. Damaging venous reflux into the skull or spine: relevance to multiple sclerosis. Med Hypotheses. 1986 Oct;21(2):141-8. (21) Carswell (1838). Pathological anatomy. Illustrations of the elementary forms of disease. London. (22) Allen (1981). Demyelinating diseases. The pathology of multiple sclerosis: fact, fiction and hypothesis. Neuropath and Applied Neurobiology 7, 169. (23) Fog (1963). On the vessel-plaue relations in the brain in multiple sclerosis. (24) Fog (1948). Rygmarvens patologiske anatomi. Munkgaards, Copenhagen. (25) Corday (1953). Cerebral vascular insufficiency. Arch of Neur and Psychiat 69, 551-570. (26) Cekic and Stein (2010). Traumatic Brain Injury and Aging: Is a Combination of Progesterone and Vitamin D Hormone a Simple Solution to a Complex Problem? NeuroTherapeutics, 7; 81-90. (27) Nemerovski et al. Phamacotherapy 29: 691- 708; 2009. (28) Ge et al. Diminished visibility of cerebral venous vasculature in multiple sclerosis by susceptibility-weighted imaging at 3.0 Tesla. JMRI 29; 1190-1194. (29) Juurlink (1998). The multiple sclerosis lesion: initiated by a localized hypoperfusion in a central nervous system where mechanisms allowing leukocytre infiltration are readily upregulated? Medical Hypotheses: 51: 299-303. (30) Kell DB. Iron behaving badly: inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases. BMC Med Genomics. 8;2:2. (31) Thomas and Jankovic (2004). Neurodegenerative disease and iron storage in the brain. Current opinion in Neurology. 17: 437-442. (32) Sullivan (2004). Is stored iron safe? J Lab Clin Med 144: 280-284. (33) Patt et al (1990). Iron depletion or chelation reduces ischemia/reperfusion induced edema in gerbil brains. J of Pediatric Surgery 25; 24-228. (34) Grant et al (2003). Iron-deficient mice fail to develop autoimmune encephalomyelitis. J Nutr. 133: 2635-2638. (35) Derfuss (2009). Contactin-2/TAG-1-directed autoimmunity is identified in multiple sclerosis patients and emdiates gray matter pathology in animals. PNAS 106: 8302-8307. (36) Rudick and Trapp (2009). Gray matter injury in multiple sclerosis. N Engl J of Med 361:1505-1506. (37) Minagar et al (2006). Multiple sclerosis as a vascular disease. Neurological Research 28: 230- 235. (38) Minagar et al (2003). Interferon beta 1a and 1b block IFN gamma induced disintegration of endothelial junction integrity and barrier. Endothelium 10:299-307. (39) Avolio et al (2005). Serum MMP-9/TIMP-1 and MMP-2/TIMP-2 ratios in multiple sclerosis: relationships with different magnetic resonance imaging measures of disease activity during IFN-beta 1a treatment. Multiple Sclerosis 11: 441-446. (40) Kraus et al (2005). Interferon-beta stablizes barrier characteristics of brain endothelial cells in vitro. Ann Neurol 56: 192-205. (41) Forsting et al (2003). Intracranial vascular malformations and aneurysms. From diagnostic work-up to endovascular therapy. (42) Djindjan (1978) Superselective angiography of the external carotid artery. Springer, Berlin Heidelberg New York. (43) Terada et al (1994) Development of acquired arteriovenous fistulas in rats due to venous hypertension. J Neurosurg 80:884-889. (44) Singh et al. (2001) Dural Arteriovenous fistula associated with prothrombin gene mutation. J Neuroimaging 11:319-321. (45) Kraus et al. (2000) Significantly increased prevalence of factor V Leiden in patients with dural arteriovenous fistulas. J Neurol 247:521-523. (46) Watanabe et al (1984) Two cases of dural arteriovenous malformation occurring after intracranial surgery. Neuroradiology 26:375-380. (47) Lawton et al. (1997) Redefined role of angiogenesis in the pathogenesis of dural arteriovenous malformations. J Neurosurg 87:267-274. (48) Marks at al. (1990) Hemorrhage in intracerebral arteriovenous malformations: angiographic determinants. Radiology 176:807-813. (49) Turjmann at al. (1995) Correlation of the angioarchitectural features of cerebral arteriovenous malformations with clinical presentation of hemorrhage. Neurosurgery 37:856-860. (50) Nataf et al. (1997) Angioarchitecture associated with hemorrhage in cerebral arteriovenous malformations: a prognostic statistical model. Neuroradiol 39:52-58. (51) Bitoh et al (1979) Spontaneous cure of dural arteriovenous malformation in the posterior fossa. Surg Neurol 12:111-114. (52) Chaudhary et al. (1982) Dural arteriovenous malformation of the major venous sinuses: an acquired lesion. AJNR Am J Neuroradiol 3:13-19. (53) Lasjaunias et al. (1984) Endovascular treatment of pure spontaneous dural vascular malformations. Review of 23 cases studied and treated between May 1980 and October 1983.Neurochirurgie 30:207-223. (54) Meder et al. (1995) Spontaneous disappearance of a spinal dural arteriovenous fistula. AJNR Am J Neuroradiol 16:2058-2062. (55) Luciani et al. (2001) Spontaneous closure of dural arteriovenous fistulas: report of three cases and review of the literature. AJNR Am J Neuroradiol 22:992-996. (56) Miki et al. (1988) Matas procedure in the treatment of spontaneous carotid cavernous sinus fistula: a complication of retinal hemorrhage. No Shinkei Geka 16:971-976. (57) Suzuki et al. (1989) Development of central retinal vein occlusion in dural carotid-cavernous fistula. Ophtalmologica 199:28-33. (58) Cognard et al. (1995) Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology 194:671-680. (59) Hamada et al. (2000) Histopathological study of venous aneurysms in patients with dural arteriovenous fistulas. J Neurosurg 92:1023-1027. (60) Borden et al.(1995) A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg 82:166-179. (61) Awad et al. (1990) Intracranial dural arteriovenous malformations: factors predisposing to an aggressive neurological course. J Neurosurg 72:839-850. (62) Davies et al (1997a) The natural history and management of dural arteriovenous fistulae, part 1. Benign lesions. Intervent Neuroradiol 3:295-302. (63) Davies et al (1997b) The natural history and management of dural arteriovenous fistulae, part 2. Aggressive lesions. Intervent Neuroradiol3:303-311. (64) Farb et al (2003) Idiopathic intracranial hypertension: the prevalence and morphology of sinovenous stenosis. Neurology 60: 1418-1424. (65) Corbett et al (2002) Idiopathic intracranial hypertension: an answer to the "chicken or the egg". Neurology 34: 370-380. (66) Higgins et al (2002) Venous sinus stenting for refrarctory benign intracranial hypertension. Lancet 359: 228-230. (67) Agid et al (2008). Imaging of the intracranial venous system. The Neurologist 14: 12- 22. |
